
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
How Cryogenic Oxygen Nitrogen Plant?
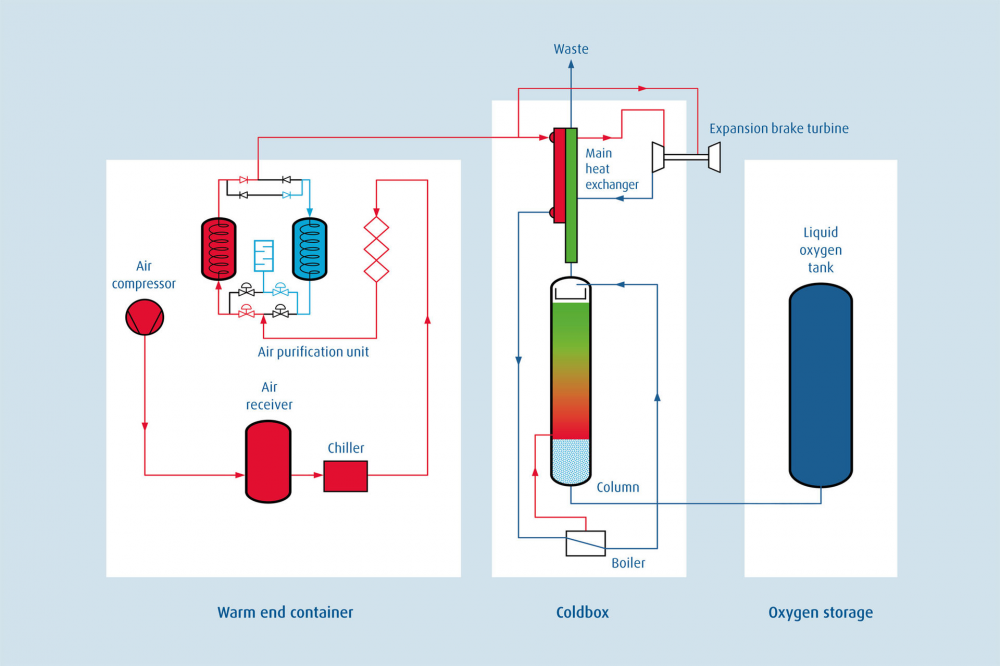 The atmospheric air mainly consists of oxygen and nitrogen gases and small quantities of water vapor, carbon dioxide, argon, helium, etc, Oxygen and Nitrogen from the air are separated due to the difference in boiling points by distillation through a fractional column.
The atmospheric air mainly consists of oxygen and nitrogen gases and small quantities of water vapor, carbon dioxide, argon, helium, etc, Oxygen and Nitrogen from the air are separated due to the difference in boiling points by distillation through a fractional column.
Atmospheric air is sucked in by a multi-stage compressor through a filter and compressed to the design pressure. The compressed air is then passed through inter-coolers, an Industrial refrigerator, moisture separators, and then to the Molecular Sieve Battery for removal of Carbon dioxide, Hydrocarbons, and moisture from the process air. This pure air then passes through the first heat exchanger, where it is cooled by the outgoing Nitrogen and Oxygen. Part of this cooled air then passes through an Expansion Engine and the other part through the 2nd heat exchanger. Both the Expansion Engine and 2nd heat exchanger help in further cooling down the air, which is finally released to the bottom of the column through an expansion valve. The air becomes liquid at this stage.
The column consists of two parts. Lower column and Upper column. In between the lower and upper column, there is a condenser that acts as a reflux for the lower column and as a re-boiler for the upper column. The liquid air at the bottom of the lower column separates through the trays of the column to give crude oxygen at the bottom and approximately 90% pure Nitrogen at the top. Crude oxygen tread as the rich liquid is then expanded through an expansion valve from the lower column to the middle of the upper column. Crude Nitrogen termed the poor liquid is expanded through another expansion valve from the top of the lower column to the top of the upper column. Due to the difference in the boiling points, the pure Nitrogen boils over and accumulates at the top of the upper co, column a, and oxygen (which has a higher boiling point than Nitrogen) accumulates at the bottom of the upper column.
Both Nitrogen and Oxygen are removed through separate paths in heat exchangers, for cooling the incoming air. Oxygen is compressed to a prescribed settled pressure by a liquid pump and is directly filled into cylinders. Nitrogen is however available at a pressure of approximately 0.5kg /cm2 and the same can be compressed into cylinders with help of an independent high-pressure compressor.
Normally the Nitrogen available is of 96 – 98% purity only and is let out in the atmosphere as a waste product. However, when nitrogen is to be filled, into the cylinders for commercial use, the plant is controlled by a change of the valve setting for making the mixed air chamber operative for achieving the required purity of Nitrogen.

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.